Innovative drug development covers more than just the substance
Patents granted for the first intracranial application system in Europe, the USA, Canada and Asia for a permanent and ambulant administration of drugs directly into the brain using CED
The two sides of the blood-brain barrier – causing 98% of potential neurotherapeutics to fail
There is a physiological diffusion barrier, which controls the exchange of substances between the circulating blood and the brain in order to protect the sensitive brain, ensuring a stable environment in the brain tissue. High molecular, charged or polar substances require specific transport systems in order to reach the brain – an effective protection, which however means that the blood-brain barrier blocks about 98% of potential neurotherapeutics. 1 “The difficulty of administering a therapeutic dose of a pharmacologically effective substance into the brain tissue is one of the main reasons for the currently very few therapy options we can offer to brain tumor patients “, says Dr. Karl-Hermann Schlingensiepen, Chief Executive Officer of Antisense Pharma. “There is only a very small number of substances capable to pass the blood-brain barrier so that they can be administered orally or by means of intravenous infusion“.
Convection Enhanced Delivery (CED) for optimum penetration of tissue
While an (intraventricular or intrathecal) injection or the placement of a drug carrier in the brain tissue circumvents the blood-brain barrier, however the regional diffusion of the drug is very limited. The concentration of the administered substance is therefore high in the direct vicinity of the place of injection, but it rapidly decreases with increasing distance. In contrast, the new application system by Antisense Pharma uses a special infusion technology. Convection Enhanced Delivery (CED) provides a high and uniform concentration of active substances in the brain tissue – even at a distance from the place of infusion. The substance is administered directly into the tumor or the surrounding brain tissue using a permanent, pressure-supported infusion using a special catheter/pump system. Since CED builds up a pressure gradient, a clearly higher and more homogeneous penetration volume can be achieved compared to conventional diffusion. The substance penetration depends on the tissue density, catheter diameter, flow rate, and others. 2
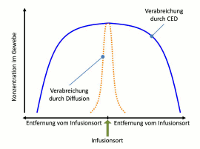
Figure: Concentration profiles of substances at a distance from the place of infusion for pressure-controlled and diffusion-controlled infusion (CED vs. injection), adapted from Raghavan et al.3
Intracranial use of CED – a high-precision technology
Originally developed at the National Institutes for Health (USA), the potential of the CED technology has now been documented by numerous animal studies, clinical studies and state-of-the-art imaging systems.3,4,5,6 This technology offers new application options for new and also for already approved substances – but its clinical use requires a suitable application system: “A CED-based intracranial infusion is a challenge for the pump technology used. Our brain is enclosed by solid cranial bones. The available space tolerates only minor infusion volumes which have to be infused smoothly using a constant pressure “, Dr. Hubert Heinrichs, Chief Medical Officer at Antisense Pharma explains. This mode of administration has so far required the use of stationary application systems and the patients had to be hospitalized for the complete therapy, involving an increased risk of infection. With the development of the first portable, now patented CED application system, the German entrepreneurs are cutting edge: “The option of a CED-based treatment using a portable application system over weeks and months could well stimulate the development of urgently required neurotherapeutics “, Dr. Heinrichs takes a look ahead.
Antisense Pharma develops first portable CED application system for the ambulant intracranial administration of neurotherapeutics
Trabedersen, the drug developed by Antisense Pharma to combat malignant brain tumors, is also not able to pass the blood-brain barrier. “Earlier examinations of brain tumor cells had already shown the very high efficacy of trabedersen“, Dr. Schlingensiepen remembers. “Due to the molecular structure, it was obvious that we would have to administer the substance directly into the brain for an extensive period of time. It is Antisense Pharma’s declared philosophy to allow critically ill people not only a longer life expectancy, but also a clearly improved quality of life. We have therefore developed in parallel to the compound also a very patient-friendly application system“.
The infusion system which has now been patented consists of carefully harmonized components: after the catheter has been stereotactically placed in the tumor, the drug is administered via an infusion line using an external, programmable, portable pump. The implantation of the catheter is a neurosurgical intervention, which is geared to the placement of a shunt for hydrocephalus patients where excessive brain liquid is diverted into the blood circulation. “The new application system uses the innovative approach so that a known and secure technology is used successfully in a completely new clinical context“, Dr. Heinrichs explains. The brain catheter is inserted through the skin to a port chamber which is usually placed in the chest muscle. The chamber is then connected to an external infusion line, which in turn is connected to a small, portable infusion pump that can easily be attached to the belt.
|
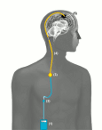
External parts: The portable infusion pump (1) is
connected with an infusion line (2). The infusion line is attached to a port needle which is inserted through the skin
into the port chamber (3) at the start of each new infusion.
Implanted parts: The port chamber (3) is implanted under the
skin on the front of the chest and connected to the
port catheter (4). The port catheter is placed under the skin
and connected with the intratumoral catheter (5). The tip
of the intratumoral catheter ends in the brain tumor.
|
 |
“Using this application system, we are able to administer highly effective drugs, by-passing the blood-brain barrier. At the same time, we allow patients to continue living their normal lives during the therapy: once the catheter system has been positioned, no extensive hospitalization is required. The total 5-month treatment using trabedersen provides patients with maximum mobility and quality of life“, Dr. Schlingensiepen summarizes.
About Antisense Pharma GmbH
Antisense Pharma is a biopharmaceutical company located in Regensburg, Germany. The company focuses on targeted therapies for malignant tumors and is dedicated to discovering and developing drugs based on antisense technology for worldwide commercialization. The medications specifically block the synthesis of key cancer proteins. Antisense Pharma has clinical trials running that involve patients with brain tumors, advanced pancreatic carcinoma, malignant melanoma and colorectal carcinoma. Therapies for other indications are under preclinical development. The company has been honored with the German Founder‘s Award and the Bavarian Innovation Award and received the Innovation Prize TOP 100.
About Trabedersen (AP 12009) and TGF-β2
Trabedersen is a first-in-class gene silencing antisense compound – a phosphorothioate oligodeoxynucleotide – designed to selectively downregulate the production of transforming growth factor-beta 2 (TGF-β2) at the translational level. TGF-β2 plays a pivotal role as a multimodal cytokine by regulating key mechanisms of tumor progression. Immunosuppression, invasion and metastasis, proliferation and angiogenesis are simultaneously promoted by TGF-β2 in a variety of malignant tumors. Therefore Trabedersen is a targeted multimodal therapy and one of the very promising immunotherapeutic approaches in the oncological field. Besides in high grade glioma, trabedersen is also being investigated in other aggressive cancers which over-express TGF-β2: Trabedersen is being systemically administered intravenously (i.v.) in adult patients with advanced pancreatic carcinoma, malignant melanoma, or advanced colorectal carcinoma in an Phase I/II study.
About High-Grade Glioma
Anaplastic astrocytoma (AA) and glioblastoma multiforma (GBM) are the two most common forms of primary brain tumors and are diseases with high unmet medical need. Adults as well as children may be affected, although the peak age is 45-65 years. Current therapies comprise surgery, radiation and/or chemotherapy. Despite recent advances, the prognosis for these patients is still poor, with a high proportion dying within two years after initial diagnosis. Antisense Pharma is the sponsor of the pivotal international SAPPHIRE clinical Phase III study, investigating the efficacy and safety of trabedersen (AP 12009) in adult patients.
Results of the Phase IIb study with trabedersen
The completed clinical Phase IIb study AP 12009-G004 was an open-label, randomized, active-controlled, parallel-group dosefinding study to evaluate the efficacy and safety of two doses of trabedersen (AP 12009) in adult patients with recurrent or refractory high-grade glioma. At 29 international clinical centers, 134 evaluable patients (39 with recurrent or refractory anaplastic astrocytoma, AA, WHO grade III and 95 with recurrent or refractory glioblastoma, GBM, WHO grade IV) were randomized to three arms: 10 μM trabedersen, 80 μM trabedersen or standard chemotherapy (temozolomide or PCV) as active control. Analysis of the core phase revealed long-lasting tumor responses and life extension in AA and GBM patients, by far exceeding the active treatment period with trabedersen. For recurrent or refractory anaplastic astrocytoma patients, median survival times in the 10 μM trabedersen group were 39.1 months compared to 21.7 months in the standard chemotherapy control arm, translating to a survival benefit of 17.4 months for patients receiving the antisense treatment over standard chemotherapy. 83.3% of the patients with recurrent anaplastic astrocytoma who received 10 μM trabedersen survived two years or more, whereas only 41.7% survived two years in the control arm with standard chemotherapy. Both efficacy and safety results have demonstrated, that the 10 μM concentration of trabedersen was superior to the 80 μM concentration. This further underlines the specificity of this targeted therapy since for optimally targeted therapies maximum tolerated dose is not necessarily the most efficient dose. This data have been published in the official journal of the American Society for NeuroOncology (SNO) in 2010 (Bogdahn U et al. Targeted therapy for high-grade glioma with the TGF-β2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro Oncol, 2010, doi:10.1093/neuonc/noq142).
Contact
Carolin Nolte
ANTISENSE PHARMA GmbH | Josef-Engert-Str. 9 | 93053 Regensburg, Germany
Phone + 49 941 920 13 175 | Fax +49 941 920 13 29 | This email address is being protected from spambots. You need JavaScript enabled to view it.
Phone + 49 941 920 13 175 | Fax +49 941 920 13 29 | This email address is being protected from spambots. You need JavaScript enabled to view it.
1 W. M. Pardridge: Blood-brain barrier drug targeting: the future of brain drug development. In: Mol Interv 3, 2003, S. 90–105. PMID 14993430 (Review)
2 Bobo RH, Laske DW et al: Convection-enhanced delivery of macromolecules in the brain. PNAS USA
3 Raghavan R. et al. Convection- enhanced delivery of therapeutics for brain disease, and its optimization. Neurosurg Focus 20(4): E12, 2006
4 Lonser R. R. et al. Successful and safe perfusion of the primate brainstem: in vivo magnetic resonance imaging of macromolecular distribution during infusion. Journal of Neurosurgery 97:905-913, 2002
5 Vogelbaum M. A. Convection enhanced delivery for treating brain tumors and selected neurological disorders: symposium review; J Neurooncol. DOI 10.2007/s11060-006-9308-9



