Bascharage, Luxembourg, December 11, 2012 / B3C newswire / - Cellon SA announced today that their entire range of PharmaTainer™ bottles and carboys are now certified “clean,” meeting USP 788 Particulate Matter in Injections, and the equivalent EP and JP quality standards.
Pharmatainer™ products are currently manufactured in PC and PET in sizes from 125ml to 20 litres. Cellon opened its state-of-the-art production moulding facility in Luxembourg in 2011. The plant is 100% dedicated to production of clean, single- use products for use in the vaccine and bio-processing sector, and operates under an ISO 9001-2008 quality system.
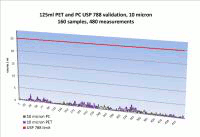
Caption: 10/25 Micron particulate in 125 mL bottle vs. limit.
(For high resolution picture please right click the image and select "Save target as...")
PharmaTainer™ carboys and bottles are manufactured in an ISO class 5 (class 100) environment and sterilised by irradiation to a SAL of 10-6. Certification to the USP 788 is based on liquid particle count analysis on a lot by lot basis.
Designed specifically for the storage and transport of bulk vaccines, biopharmaceuticals, bulk intermediates and other biotech materials, PharmaTainer™ products also provide tamper evident packaging and are first in the industry to provide bottle labelling for individual bottle traceability and inventory control.
Speaking for Cellon, Managing Director Richard Fry said, “The PharmaTainer™ range addresses directly the main quality concerns for single-use storage and transport containers in the industry, i.e. particulate and traceability. By dedicating our facility to production of clean products, we ensure that all our products are made to a single, high standard. We reduce risk by manufacturing for a single purpose and quality standard. So, there is less opportunity for error in our operations than in multipurpose moulding facilities that manufacture products of differing levels of cleanliness using multiple grades of materials.
We welcome customers and prospective customers to visit and/or audit our facility so they may see first-hand the quality systems we have in place”.
For more information on particulate testing and PharmaTainer™ cleanliness, visit www.cellon.lu or www.biofluidfocus.com.
About Cellon
For 25 years, Cellon has provided products and services to the biopharma and vaccine manufacturing industries in Europe. Innovation, quality and customer service have made Cellon a trusted vendor for many of the world’s leading biopharma and vaccine manufacturing companies. Sterile, single-use products and tissue culture apparatus are areas of special focus.
About Biofluid Focus
Biofluid Focus is a distributor of plastic products for biotechnology industries and is the exclusive North American distributor of PharmaTainer™ products. Staff members are experienced in manufacturing processes and quality requirements for a broad range of single-use products, including tissue culture labware and containers.
Contact
Richard Fry
Cellon SA
ZAE Robert Steichen
16, rue Hèierchen
L-4940 Bascharage
G.D. Luxembourg
Phone: 352-263-373
Email: This email address is being protected from spambots. You need JavaScript enabled to view it.