- Anti-midkine antibodies reduced mortality rate and preserved kidney function in a mouse model of diabetic nephropathy
- Kidney damage markedly reduced in treated animals
- Additional data bolsters preclinical antibody data package
- Large unmet medical need for patients with kidney disease
Sydney, Australia, January 24, 2013 / B3C newswire / - Cellmid Limited (ASX: CDY) has completed its first in-life diabetic nephropathy study with the Company’s anti-midkine antibodies (MK-Ab) in a mouse model of the disease. Two of Cellmid’s proprietary MK-Ab’s were tested. Both antibodies reduced kidney damage significantly, as assessed by functional and histological analysis, with kidney structure largely preserved in the treated animals.
This study provides important new information, as it is the first time the Company has used its own MK-Ab’s in a therapeutic setting in a kidney disease model.
Renal histological assessment showed that glomerular sclerosis was reduced from 48% in untreated animals to below 20% in both MK-Ab treated groups (p<0.01). Interstitial volume was also significantly reduced, from 35% in untreated animals to 12% in both antibody groups (p<0.01). MK-Ab treatment also maintained tubular cell height; untreated animals had mean cell heights below 2μm, compared to 4μm for treated animals (p<0.05).
Kidney function was also preserved, with MK-Ab treated animals showing reduced protein leakage into the urine compared to untreated controls. Protein casts in the kidney, indicating damage, were also significantly reduced in antibody treated animals (Figure 1). Importantly, the MK-Ab treated animals showed healthy weight gain and reduced mortality compared to untreated controls; only 6.3% of treated animals died before the end of the study, compared to 25% of the untreated animals.
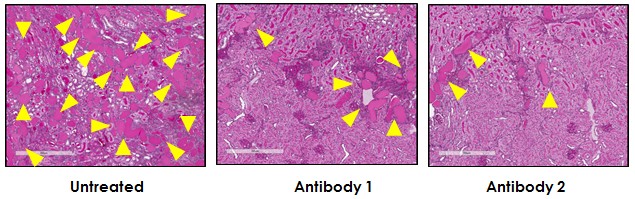
Figure1. Anti-MK antibodies reduce protein cast deposits in the kidneys of mice with AN-induced nephropathy.
Photographs show representative histological sections from treated and untreated mice. Protein casts are bright pink; yellow arrows indicate large protein cast deposits.
Midkine’s role in kidney disease has been extensively studied in the past and is the subject of a dozen peer-reviewed publications. These studies show that MK is a key driver of inflammation and damage in a variety of kidney disease and injury settings.
The current study using Cellmid’s MK-Ab’s was conducted by scientists at the Centre for Transplantation and Renal Research (CTRR), based at the Westmead Millennium Institute and University of Sydney, Westmead Hospital, using an Adriamycin (AN)-induced mouse model of nephropathy. In this model, a single AN injection leads to kidney damage reminiscent of that seen in human diabetic nephropathy.
Diabetic nephropathy is the leading cause of chronic kidney disease globally. It is also one of the most significant long-term complications in terms of morbidity and mortality for patients with diabetes. In the USA alone, diabetes affects 26 million people, and the US Centre for Disease Control (CDC) estimates that as many as one in three adults could have diabetes by 2050 if current trends continue.
Currently, diabetic nephropathy is managed by keeping glucose levels under control, however many of the patients develop end stage renal disease (ESRD). It is estimated that 30-40% of all ESRD is caused by diabetic nephropathy.
ESRD requires the traumatic and costly interventions of kidney dialysis or transplant. A treatment that slowed or halted the progression of diabetic nephropathy into full-blown ESRD would have enormous benefits for the quality of life of diabetes sufferers in addition to reducing the massive costs associated with the treatment of ESRD.
A 2010 report by Kidney Health Australia estimated that dialysis costs between A$53,000 and A$79,000 per patient per year. The same report costed kidney transplants at A$81,000, with nearly A$12,000 per patient per year in ongoing costs (in 2008-9 dollars; http://www.kidney.org.au/LinkClick.aspx?fileticket=i759hVXpJI0%3D&tabid=635&mid=1837 ).
The results of this diabetic nephropathy study present a promising start to the Company’s review of the therapeutic potential of its anti-MK antibody portfolio. It will contribute to the decision to select a lead disease indication Cellmid can then take into the clinic.
About Cellmid Limited (ASX: CDY)
Cellmid is an Australian biotechnology company developing innovative novel therapies and diagnostic tests for inflammatory diseases, heart attack and cancer. Cellmid holds the largest and most comprehensive portfolio of intellectual property related to midkine and midkine antagonists globally. The Company’s most advanced development programs involve using its anti-midkine antibodies for the treatment of cancer as well as inflammatory and autoimmune disorders. In addition, Cellmid is commercialising midkine as a biomarker for cancer diagnosis. Elevated midkine concentration in the blood and other body fluids is strongly indicative of cancer. Cellmid’s first product, the MK-ELISA, is a blood test that sensitively and accurately measures serum midkine levels.
About Midkine (MK)
Midkine is a multifunctional growth factor that is highly expressed during embryonic development. Midkine modulates many important biological interactions such as cell growth, cell migration and cellular adherence. These functions are relevant to cancer, inflammation, autoimmunity, ischemia, nerve growth/repair and wound healing. Midkine is barely detectable in healthy adults and only occurs as a consequence of the pathogenesis of a number of different disorders. Midkine expression is often evident very early in disease onset, even before any apparent physical symptoms. Accordingly, midkine is an important early marker for diagnosing cancers and autoimmune diseases. Finally, because midkine is only present in a disease context, targeting midkine does not harm normal healthy tissues.
Contact:
Maria Halasz, CEO
T +612 9299 0311


