A-SKIN seeks financing or business deal on preclinical studies with WHF and Tiscover (clinical phase II)
- High quality regenerative solution for patients with difficult to heal wounds – worldwide market estimated at $20.5 billion by 2020
- Swift follow-up research to bring WHF® into the clinic and patients
- WHF® second lead product to bring to the clinic after the skin substitute Tiscover® produced from patient's own skin cells
- Company in process of next financing round for early clinical study of WHF and phase II clinical trial with Tiscover®
- Compelling portfolio of three skin engineering therapies developed within the company, linked to Amsterdam VU University Medical Center
- Tiscover® and WHF® are patented
Amsterdam, The Netherlands, January 13, 2017 / B3C newswire / -- A-SKIN, a leading Dutch skin tissue engineering biotechnology company, developing new high quality cultured skin therapies for difficult to heal skin wounds, has secured a very important patent for its groundbreaking Wound Healing Formulation® therapy. The worldwide market for this indication is estimated at $20,5 billion by 2020. Furthermore A-SKIN’s lead product Tiscover® is ready to enter a clinical phase II trial after finalization of an ongoing financing round to raise € 7,5 million.
WHF®, short for Wound Healing Formulation®, is a substance which can be directly applied on a wound to stimulate the wound healing. WHF consists of wound healing factors secreted by cultured immortalized human skin cells, with the potential to vitalize the natural human wound healing process. WHF substance can be applied directly on the wound bed in a gel, blister, bandage etc. The target population is every one with a small simple skin wound which needs wound healing stimulation till small, large hard to heal wounds, for instance diabetic, venous and trauma. WHF is classified as a Non-Cell Based Medicinal Product at the European Medicine Agency (EMA).
Prof. dr. Rik J. Scheper, A-Skin’s CEO, comments:
|
|
“Securing the IP of WHF means a very important milestone for our company, with now two ground breaking skin engineering products in our pipeline. Both WHF and Tiscover tap into a huge worldwide market for regenerative skin engineering medicine and non-healing chronic wound treatment. Some 50 million patients around the globe suffer from non-healing wounds following higher incidence of diabetes, ageing and growing immobilization. Therefore our solutions have a large societal impact by healing patients wounds, taking away the pain and giving back their self-esteem.” |
WHF®
The preclinical research on WHF® was an EU-subsidized collaboration between A-SKIN and Amsterdam VU University medical center. To conduct the research and proceed in the near future master and working cell banks of the cell lines were established. All cell banks are stored in liquid nitrogen for further research. The cell lines underwent several culture medium switches. First they were cultured in their “original medium” as described by the delivering company, followed by A-Skin’s optimal “research grade medium”, finally replaced by clinical grade medium. The project has generated a novel Wound Healing Formulation (WHF) based on the secretome of a co-culture of keratinocyte and fibroblast cell lines. The WHF increases wound-closure predominantly by stimulation of fibroblast and keratinocyte migration.
Method
For the production of WHF® a patented method for the preparation of a complex secretion is used. This comprises the steps of a) co-culturing defined numbers of immortalized human fibroblasts and keratinocytes, thereby producing an optimal mélange of wound healing mediators; and b) separating the secretion from the fibroblasts and keratinocytes. In this way, a very potent wound healing formulation (i.e. the secretion) is obtained which can be used for the treatment of a variety of skin wounds.
Tiscover®
The lead product of A-SKIN, Tiscover®, is cultured autologous full thickness skin aimed at the healing of chronic, therapy-resistant wounds. Tiscover focuses on a niche market of around € 200 million in the US and €100 million in Europe. In Benelux and Germany 1 million patients suffer from a leg wound of which at least 20% become chronic. With an initial market share of 2.5% over 5000 patients can be treated with Tiscover in Benelux and Germany alone. Tiscover® is an innovative skin substitute, produced under cGMP from the patient's own skin cells using a patented tissue engineering technology.
Method
The technology involves isolating cells from a small strip of the patient's skin (one or more 3 mm diameter skin biopsies) and isolating, nurturing, and expanding them (cell culturing) under specialized conditions in the dedicated cleanroom laboratory of A-SKIN. This process results in a 10-20 fold amplification of the initial skin biopsies, to a full thickness skin patch with both dermal and epidermal layers. Thus, Tiscover is an autologous Advanced Tissue Medicinal Product, ATMP (EU Regulation (EC) No 1394/2007). Tiscover is classified (by EMA) as a Tissue Engineered Product (TEP). The interaction of autologous dermal and epidermal cells ensures abundant secretion of chemokines, growth factors and cytokines into the non-healing wound bed. Wound repair is further facilitated by direct cover of the exposed area.
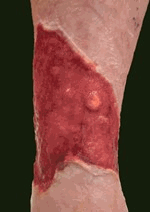
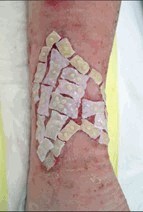
| Tiscover® treatment | ||
 |
 |
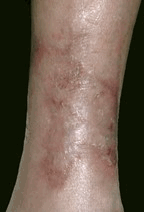 |
| Before | Application | Healed |
Clinical Phase I Results and start of Phase II with Tiscover® after financing round
In early Phase I clinical studies in total 66 wounds after single-skin substitute applications were assessed with follow up. The trial data showed that the application of Tiscover® is safe, can reactivate the inert wound bed and results in a significant improvement of chronic skin wound healing in patients refractory to conventional (compression etc.) therapies (see Blok et al, 2013). In the meantime A-SKIN set up a compelling design for a phase II non-blinded randomized multicenter Tiscover trial for further safety and efficacy data. This trial is scheduled to broaden to Germany after completion of an ongoing financing round to raise in total € 7,5 million. The Tiscover trial will be performed under cGMP and GCP.
The global wound care market
An estimated 40 million chronic wound (leg, pressure and diabetic) patients worldwide and 5 million new patients in EU need more advanced wound care each year. Open leg/foot wounds are the most common type of wounds, accounting for 70-90% of all wounds. An estimated 15 % (1.2 million) of patients with diabetes will ultimately develop diabetic foot wounds. Additionally, each year some 2 million people develop pressure wounds in Western society. At least 20% of the wounds are still open after 2 years and are chronic.
The global wound care market is expected to reach $18.3 billion by 2019 from $15.6 billion in 2014, growing at a compound annual growth rate (CAGR) of 3.2% from 2014 to 2019. Based on the type of products, the wound care market is segmented into traditional wound care, basic wound care, advanced wound care, bio-active wound care, and therapy devices. The advanced wound care segment commanded the largest share in 2014, while the bio-active wound care segment is forecasted to be the fastest-growing (10% per year). Factors such as rising awareness regarding new technologies for wound care, government support in the form of funding, rising diabetic and aging population are driving the growth of the wound care market.
About Wound Care
Chronic open wounds form an important societal and economical challenge. Non-healing chronic wounds are open wounds accompanied by loss of skin and/or surrounding tissue and do not heal within 3 months despite optimal standard wound care in line with Dutch, EU and US guidelines. These wounds occur with high incidence (1-2% in the Western population) accounting for a total of 50 million patients globally. The main causes for these open wounds (also known as ulcers) are: venous / arterial insufficiency, diabetes, age and pressure due to continuous immobilization (wheelchair use or bed confinement).
These wounds have a major impact on patient wellbeing due to continuous pain, risk of infections, discharge of smelly fluids, and reduced mobility, indicating the high societal impact of this condition. The labour and cost intensive wound care needed for these patients represents also a substantial economic burden and has significant impact on the healthcare costs and sustainability.
For example, in the UK, chronic open leg wound patients alone take up to 50% of the working time of community nurses and around 80% are treated at home. The annual costs of a chronic open venous leg wound have been estimated on € 10.000 – 25.000 annually.
The conventional therapies for treating open wounds include non-curative measures as wound cleansing, compression therapy (venous leg ulcers), pressure off loading (pressure ulcers), application of moist- and antimicrobial wound dressings, and more advanced, hospitalized treatments such as Vacuum Assisted Closure, placing skin biopsies or skin auto-grafting. Standard wound therapies have proven inadequate in many chronic, hard to heal cases. For instance conventional treatments for venous leg wounds are effective in only 50% of the cases and take up to 4 months.
Out of the remaining non-healing wounds, 20% are still open after 2 years, and 8% are still open after 5 years. Furthermore, wound recurrence is high with 30-60% recurrence in the first year. It is clear that novel wound-healing strategies for the treatment of these hard-to-heal wounds are urgently needed.
About A-SKIN
It was founded in 2006 by the VU University Medical Center (VUmc), departments Dermatology and Pathology. The continuous unique interaction within the A-SKIN team of regenerative research, scientists and clinicians enables A-SKIN to bring cultured skin constructs to the clinic for advanced wound therapies and difficult skin defects. A-SKIN has a strong academic basis in regenerative medicine, skin tissue engineering, allergic contact dermatitis, and compound screening for irritancy and allergenicity.
Management
Prof. dr. R. (Rik) J. Scheper, CEO, Chairman of the Board
(Co-)founder and active member of the European Research Group on Experimental Contact Dermatitis. (Co)founder and member of the European Environmental Contact Dermatitis Research Group. To further develop his work on human dendritic cell lines in allergy and cancer he (co-) founded DC-Prime BV aiming at novel tumor vaccines. Currently he serves DCPrime as member of the Scientific Advisory Board.
Prof.dr. S. Gibbs, Director Research & Development
Prof. Dr. Gibbs obtained her PhD in molecular genetics in 1991 in Leiden. In 1993 she became head of the Dermatology research laboratory in VUMC, Amsterdam. The development and application of cultured skin and oral mucosa is her major interest. Her entire career has focused on human skin biology, and in particular in animal alternative methods to develop novel therapeutic strategies for treating and preventing human skin disease.
A-SKIN focuses on developing human skin models and topical applications for advanced Wound Healing Products and Skin Testing Services.
cGMP quality system
Tiscover and A-SKIN have a cGMP quality system in place (production process is fully covered in Standard Operating Procedures) and Investigational Medicinal Product Dossier (IMPD). The Dutch Inspectorate of Health (IGZ) has approved the laboratory and production process of Tiscover with a cGMP production process licensee for ATMP production.
Product Portfolio A-SKIN
A-SKIN develops advanced wound care products and therapies based on (autologous) cultured human skin cells. A-SKIN has 3 products in its portfolio.
- Tiscover®: autologous cultured human skin for chronic wound healing;
- WHF, Wound Healing Formulation: a topical application of a unique cocktail of allogeneic secreted wound healing factors to stimulate acute and chronic wound healing directly repeatedly.
- KC-Matrix: autologous cultured keratinocytes on a CE labelled carrier for healing burn wounds.
Contacts
A-SKIN
Chantal Blok
Business Development
+31 6 242 06 534
This email address is being protected from spambots. You need JavaScript enabled to view it.
Michiel Hiemstra
Chief Commercial Officer
+31 6 215 38 903
This email address is being protected from spambots. You need JavaScript enabled to view it.
LifeSpring LifeSciences Communication, Amsterdam
Leon Melens
+31 6 538 16 427
This email address is being protected from spambots. You need JavaScript enabled to view it.




