![]()
- Semaglutide is the active ingredient of weight loss medications Ozempic® and Wegovy®
- Semaglutide as an oleogel formulation using the BrainDos™ technology is internalized much more strongly than in an aqueous formulation used for example in pens
- This new formulation and intra-nasal application could help alleviate the obesity-related healthcare burden as the application costs are significantly lower than with pens
EMMETTEN, Switzerland, May 08, 2024 / Biotech Newswire / -- MetP Pharma AG, leveraging the proprietary nose-to-brain drug delivery technology BrainDos™ in internal and external product development and manufacturing programs, today announced promising preclinical research results that strongly support the further development of semaglutide in an oleogel formulation using the BrainDos™ drug delivery technology.
Semaglutide, the active ingredient of Ozempic® and Wegovy®, is an antidiabetic medication used for the treatment of type 2 diabetes as well as an anti-obesity medication used for long-term weight management. It is a Glucagon-Like Peptide-1 Receptor Agonist (GLP-1-RA) that is currently administered through subcutaneous injection using pens.
GLP-1 receptors are found on beta cells of the pancreas and on neurons of the brain. There, signal chains are activated that suppress the feeling of hunger and increase the feeling of satiety. It is proven that GLP-1 RAs can send information to the brain via neural and humoral pathways.
Preclinical studies in Cultured Human Airway Epithelial Cells (Calu-3) conducted at MetP Pharma AG demonstrate that the semaglutide oleogel in the BrainDos™ formulation leads to a 10-fold increase in tyrosine hydroxylase (TH) expression, compared to only a 2-fold increase of the aqueous semaglutide solution as used for example in pens. As obesity leads to a reduction in tyrosine hydroxylase (TH) levels (1) an increase in TH levels is a suitable biomarker for drug delivery. The results also demonstrate a significantly higher TH response at 4h than at 24h post treatment but the TH level at 24h is still about 3-fold higher than the untreated control demonstrating consistent activity. The semaglutide concentration in both the oleogel formulation and the aqueous solution was 10nM.
Dr. Claudia Mattern, Chief Scientific Officer at MetP Pharma says: “The preclinical studies clearly show that the oleogel formulation with BrainDos™ increases the delivery of semaglutide into cells. This makes BrainDos™ suitable for re-formulating active ingredients with expiring patents and re-patenting them using a new, innovative application method. It is important to understand that BrainDos™ is an innovative nose-to-brain drug delivery technology that in no way is comparable to traditional nasal sprays.”
A nasal application of semaglutide could have several advantages compared to pens such as non-invasiveness, fast and easy administration, painless application for patients with needle phobia, fewer adverse events through reduced systemic exposure of the drug, and much less costs per application than pens. In addition, there is the ability for direct CNS delivery using BrainDos™ potentially resulting in a higher bioavailability.
Obesity is a huge problem in many countries and is even classified as an epidemic by the World Health Organization (WHO). This new formulation and administration mode could help alleviate the obesity-related healthcare problem as the manufacturing costs are significantly lower than with the currently used pens and the application is more convenient.
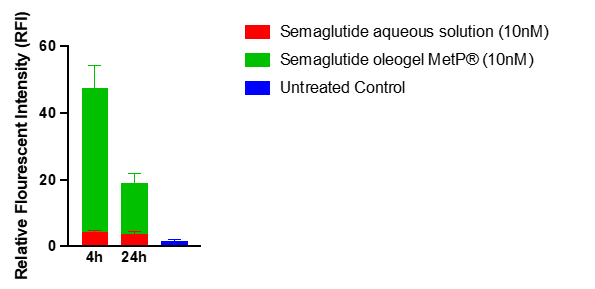
Semaglutide in the BrainDos™ formulation (green) leads to a highly significant increase in tyrosine hydroxylase (TH) expression, compared to the aqueous semaglutide solution as used for example in pens (red).
About BrainDos™
BrainDos™ is an intranasal oil-based drug delivery system developed to bypasses the blood-brain barrier (BBB) and allowing precise drug administration. BrainDos™ consists of a thixotropic oleogel, Silcum®, enclosed in a mono-dose applicator (Lecticula®). The advanced gel system incorporates the active pharmaceutical ingredient (API) within a lipid-based matrix and exhibits distinctive intranasal drug retention and mucosal adhesion properties thus allowing optimal drug release and exposure to the nasal mucosa. Silcum® facilitates the direct entry of drugs to the brain via the olfactory and trigeminal pathways as well as the topical and systemic delivery via the different tissue types in the nasal cavity. Lecticula® is a fingernail-sized mono-dose container ensuring easy application and providing high dosing accuracy (± 3%). Its supply chain is secure, there is no risk of shortage and recycling is easy. Lecticula® was among the finalists for the 2016 CPhI Pharma Award in the category "Excellence in Pharma: Drug Delivery Devices". The filling of the single-dose containers at laboratory, pilot and industrial scale are carried out in specially designed filling machines.
About MetP Pharma AG
MetP® Pharma AG is an independent, specialized pharmaceutical R&D and product development company based in Switzerland. The company has developed BrainDos™, an advanced and comprehensively patented nose-to-brain drug delivery system allowing to bypass the blood-brain barrier. The brain targeting effect of BrainDos™ has been demonstrated through various proof-of-concept studies. BrainDos™ is a fully developed and flexible technology platform that is significantly reducing the risk of pharmaceutical product development. The company’s internal product development program includes intranasal products for the indications concussion, insomnia, attention deficit hyperactivity disorder (ADHD), multiple sclerosis (MS) and hypogonadism.
MetP® also provides pharmaceutical development services based on its proprietary and worldwide patented intranasal BrainDos™ delivery technology. The company’s research has been published in 65 peer-reviewed journals and a robust intellectual property portfolio exists in a large number of countries until 2037/2039.
Contact
MetP Pharma AG
Dr. Claudia Mattern
CSO
+41-41-618 30 30
This email address is being protected from spambots. You need JavaScript enabled to view it.
Reference:
1. Gabery et. al., Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020;5(6):e133429. https://doi.org/10.1172/jci.insight.133429
Keywords: Glucagon-Like Peptide-1 Receptor Agonists; Glucagon-Like Peptides; semaglutide; Wegovy; Ozempic; Tyrosine 3-Monooxygenase; oleogels; Epithelial Cells; Biomarkers; Obesity; Drug Delivery Systems; Drug Compounding; Organic Chemicals; Drug Evaluation, Preclinical; Switzerland; GLP-1; Oleogel formulation; BrainDos™ technology; Drug delivery; Ozempic®; Wegovy®; Obesity; Preclinical research; Tyrosine hydroxylase (TH) expression; Biomarker; Nasal application; Non-invasiveness; Blood-brain barrier (BBB); Intranasal drug delivery system; Silcum®; Lecticula®; MetP Pharma AG; Dr. Claudia Mattern; Chief Scientific Officer; Product development; Brain targeting; Proof-of-concept studies; Intellectual property portfolio
Source: Biotech Newswire


