- The disposable Switch® technology allows for high precision suturing of tubular (e.g. arteries) and layered structures (e.g. skin), twice as fast as the conventional technique
- High precision and faster suturing provides benefits to patients as it reduces the risk of complications and results in a reduction of overall healthcare costs
- Mellon will tap into an annual worldwide sutures and suturing devices market of $3bn
- Mellon will introduce its technology in an 8cm Switch® model, initially focusing on three vascular indications: carotid artery surgery, kidney transplant and peripheral bypass surgery
- Further development of suturing devices platform allowing for suturing in most medical disciplines: micro surgery model, 10cm model, diversification in suture material, a laparoscopic model, and a robotic version
- Market launch (Netherlands and Flanders) scheduled for late 2017, after completion of development and CE certification process
- International market launch expected as early as 2018
- Additional funding required for market introduction and the development of its micro surgery model
Nijmegen, The Netherlands, September 30, 2016 / B3C newswire / -- Mellon Medical is unveiling a patented ground breaking global innovation in suturing technology. The company has developed a platform technology for suturing with a single hand. The Switch®, a disposable precision-suturing instrument, enables surgeons to suture tubular and layered structures about twice as fast as the conventional technique. The technology is expected to reduce the risk of complications, resulting in improved patient outcome and a reduction of costs. Mellon expects market introduction of the Switch® – which has been successfully tested by a large number of experienced vascular surgeons – in 2017, once final development and CE certification process have been completed.
Investors in the technology include Dutch Thuja Capital, PPM Oost, Brabantse Ontwikkelings Maatschappij (Brabant Development Company) and Rijksdienst voor Ondernemend Nederland (Netherlands Enterprise Agency). Besides its 8 cm model, Mellon has started the development of a micro surgery-model of the Switch®, designed for a.o. coronary artery and arteriovenous fistula procedures. Mellon is in talks with a number of investment parties for the financing of this model.
Platform technology
Mellon has developed a platform technology for suturing with one hand. The use of a straight needle in its device allows for suturing of all tubular and layered structures. Although market introduction is focused on vascular surgery, Mellon’s technology can be used in a broad range of other disciplines, like general surgery, urology, gynecology and neurosurgery. With the development of devices with varying geometries, needles and sutures and with the development of a micro surgery model, a laparoscopic and a robotic variant, Mellon can deliver the full potential of its platform.
Conventional suturing versus The Switch®
Classical suturing is a complex process and takes a long time to learn. Focus is on getting control over the needle. Surgeons using conventional suturing techniques perform several coordinated motions using both hands in order to place a single suture. The needle is positioned into the needle holder and passed through the tissue, where the needle is recovered by a forceps. The needle is then repositioned into the needle holder and the process is repeated with every suture. On average, 30% of the operation time is spent on suturing.
Mellon has managed to reinvent the technique of suturing and has already received several international innovation awards for this new invention, including one from the vascular surgeon community during this years’ Charing Cross International Symposium for Vascular Surgery in London.
The Switch® can be operated by lightly pinching the double-action buttons with thumb and index finger of one hand. The other hand is free to present the tissue to be sutured. This technique greatly improves the precision and efficiency of the suturing process, as surgeons no longer need to switch the needle between instruments and focus on getting control over the needle.
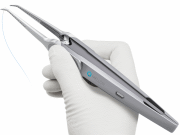

In the Switch®, the needle is always secured in one of the two jaws. The predictable linear path followed by the straight needle causes less motion friction, reducing the likelihood of damage to the vessel wall and resulting in a high quality connection.
Mark Vrancken Peeters, vascular surgeon and Mellon’s Chief Medical Officer, commenting on the innovation: “By collaborating with medical specialists, industrial designers and ergonomic experts in the development of the Switch®, Mellon has been able to reinvent suturing, bringing suturing technology into the 21st century. Our mission is to improve medical procedures by providing the best possible tools. We believe our innovative platform suturing technology will improve patient outcome and reduce overall healthcare costs.”
Easy to adapt
The Switch® is intuitive to use and the new method is easy to learn for skilled and newly trained surgeons. The comfort of one handed suturing yields more accurate suturing in less time. Once surgeons are familiar with the device, significant time is saved during a critical phase of the vascular procedure. A pilot test with an early Switch® prototype was conducted with seven vascular surgeons, after which it was concluded that performing a vascular anastomosis with the Switch® prototype is 44% faster than suturing in the classical way. Through a reduction of the arterial cross-clamping time, patient outcome is expected to improve.
Pre-clinical and clinical field studies
In the process of obtaining CE mark, Mellon will perform tests on pigs, where vascular surgeons will perform vascular anastomoses with the Switch® on the groin and carotid arteries. Besides documenting the ease of use and the fail safety, histological studies will have to show that suturing with the Switch® is more accurate and less damaging to the vessel wall.
MedCert, Mellon’s Notified Body, performed a Clinical Protocol Review on Mellon’s Pre-clinical and Clinical Regulatory Strategy. Although it is expected that no clinical trials are needed for obtaining CE mark, Mellon will start performing clinical trials after market introduction, to prove superior accuracy and the timesaving ability of its device. The safety and performance of the Switch® will be established as a result of existing research literature and the simulation of clinical practice through pig animal tests.
CE mark
Mellon will be the legal manufacturer and will apply for ISO 13485 certification within six months after closing its B-round financing which is currently underway. The Switch® and its accessories are considered to be a class III medical device (EU). MedCert (Hamburg, Germany) has been appointed as Notified Body (med-cert.com) The ‘Preclinical and Clinical Strategy’ has been reviewed by the Notified Body: no human studies are expected to be necessary to prove safety of the device in clinical use. The necessary safety tests, which include durability testing, usability testing, bench testing and animal (pig) testing will be performed by a Dutch CRO. Mellon plans to obtain CE mark within a year after closing of the B-round financing.
For FDA, Mellon anticipates a 510(k) pathway, assuming the FDA would accept a rationale that shows the technological characteristics of the Switch® are substantially equivalent to previously listed devices for general surgical purposes (Class II). Mellon expects a Premarket Approval (PMA) for the intended use in the central circulatory system (Class III in carotid artery surgery). None of the devices listed by the FDA compete with the Switch® suturing device in open vascular surgery. Clinical studies are assumed to be part of the PMA to prove safety and efficacy. Mellon plans for FDA approval within two years after obtaining CE mark.
Building a strong portfolio
Mellon will develop different dimensions of the hand-held device, to cover the whole range of vascular surgery as well as procedures in other surgical disciplines. Mellon will start with an 8 cm version for accurate suturing of blood vessels in superficial wounds, and shortly thereafter introduce an 11 cm version for delicate procedures in deep cavities (vessels of about 1 cm in diameter). Next in its product portfolio is the development of a micro-surgery model, capable of suturing smaller vessels (1 to 3 mm in diameter). Thereafter, a laparoscopic model and a reusable (electrical) model with exchangeable jaws will be developed. In parallel, Mellon intends to develop other proprietary needle-suture combinations (e.g. PDS, vicryl) that can be used in the various hand-held devices.
Visit www.mellonmedical.com for a 3D animation.
About Mellon
Mellon Medical was founded early 2013 by Lieuwke de Jong and vascular surgeon Mark Vrancken Peeters, with the objective of developing a new medical device that enables more efficient and effective suturing of tubular and layered structures. Having long been aware of the complexity of suturing such tissue through his many years of surgical practice, Vrancken Peeters strongly believed that suturing with one hand could improve the procedure and therefore patient outcome. Driven by this belief and his desire to help more than one patient at a time, he traded his career as a surgeon to achieve this mission as CMO of Mellon.
Mellon’s 8cm Switch®, was designed to accomplish the most accurate suturing in half the time, contributing to the best possible patient outcome in vascular surgery. It is the future suturing device of choice for surgeons who conduct the most challenging procedures. In essence, all tubular and layered structures can be sutured with the Switch®. It is unique, as it is the only device able to perform precise automated suturing in vascular surgery. Its main competitor is classical suturing, done with a (Castroviejo) needle holder and forceps, as both techniques use a suture thread to ligate the tissue.
Thanks to Mellon’s patent portfolio, consisting of four patent applications, the company has effectively protected both its current technology, as well as their future diversification, allowing for scalability of Mellon’s product offering.
Following a Series A funding round, investors Thuja Capital, Brabantse Ontwikkelings Maatschappij (Brabant Development Company) and Participatiemaatschappij Oost Nederland (Eastern Netherlands Private Equity Company) joined as shareholders as early as May 2013, encouraged by the highly innovative nature of Vrancken Peeters’ idea. The first innovation loan was granted by the Netherlands Enterprise Agency in 2014.
The Management Team consists of Lieuwke de Jong, Dr. Mark Vrancken Peeters – former vascular surgeon, and Jan Benschop, and combines both valuable medical expertise and corporate, commercial as well as entrepreneurial experience. The team will be strengthened with additional experts. Mellon works with committed, trusted and creative partners, with a proven track record within the medical device industry.
Mellon’s four-member Supervisory Board is chaired by Jan Hendrik Egberts, entrepreneur and advisor to the venture capital, specialty pharmaceutical and medical device sectors. He was the former CEO of Octoplus, chairman of Molnlycke Healthcare Inc., and global executive board member for Johnson & Johnson Medical.
The Supervisory Board is completed by experienced life sciences entrepreneurs:
- Rob de Ree – former CEO of Dezima Pharma, that was sold to Amgen in 2015 and of BMEye, that was sold to Edwards Lifesciences in 2012. Holds a seat in the Supervisory Board of Medisse and Ventinova Medical.
- Tijn van Beek – founder and owner of Van Beek Medical in Sneek, a major dealer in laparoscopic products ranging from camera covers to suction and irrigation systems, from disposable laparoscopic instruments to reusable ones. The company was sold to Arseus Medical in 2012.
- Michel Briejer, PhD – partner of Dutch Venture Capital company Thuja Capital. He gained experience in a variety of senior management positions, both in the international pharmaceutical industry (Janssen Pharmaceutica and Yamanouchi), as well as in the biotech industry (Crucell).
Mellon has established an esteemed international Medical Advisory Board to provide leadership on the medical aspects of its new suturing technology. Members are world wide renowned vascular surgeons:
- Prof. Willem Wisselink, MD, PhD. – Chief of the Vascular Surgery Division and Vice Chair of the Department of Surgery of VU University Medical Center, Amsterdam. He is also deputy Professor of Surgery at the University of Illinois, Chicago.
- Prof. Jean Panneton, MD, FACS, FRCSC – Professor of Surgery and Vascular Surgery Chief and Fellowship Program Director at Eastern Virginia Medical School in Norfolk, VA.
- Prof. Ralf Kolvenbach MD, PhD, FEBVS – Director of the Department of General and Vascular Surgery, Endovascular Therapy and Phlebology at Augusta Hospital and Catholic Clinics Group, Düsseldorf, Germany. He is also a Clinical Professor of Vascular Surgery at the Heinrich-Heine University of Düsseldorf, Honorary Professor Sino-Japanese Friendship Hospital and University of Beijing, China and Professor and Co-chair of the Allied Health Profession Program at the Fliedner University in Düsseldorf.
- Juan Carlos Parodi, MD, PhD – Honorary professor of Surgery at the Universidad de Buenos Aires and works as Chief of Vascular Surgery at the Trinidad Hospital, san Isidro, Buenos Aires. He was formerly a professor at the Wayne State University, the Washington University School of Medicine, and the University of Miami. He pioneered the first stent graft for the treatment of aortic aneurysms in 1990. Prof. Parodi is the recipient of numerous distinctions.
Contacts
Mellon Medical
Lieuwke de Jong, CEO
Nijmegen, the Netherlands
Tel: +31 (0)24 20 22 116
Mob: +31 (0)6 345 00156
This email address is being protected from spambots. You need JavaScript enabled to view it.
LifeSpring LifeSciences Communication
Amsterdam, the Netherlands
Leon Melens
+31 (0)6 538 16427
This email address is being protected from spambots. You need JavaScript enabled to view it.


